| hydrogen Fuel hydrogen
"Run your car on water". "Clean the air as you drive". "Do it yourself, it's cheap and easy". More than likely, you've heard these claims discussed in conversations, posted on the web, or seen them in magazine ads. If not, let Diesel Power be the first to tell you that yes, water has the potential to help power your diesel vehicle. The question becomes: is hydrogen for you, and at what cost?
Hydrogen BasicsHydrogen is the lightest, simplest (contains only one proton), most abundant gas in the universe. It is the fuel of choice for our sun, so calling hydrogen an alternative energy source is a stretch. In the 1860s and 1870s, some of the first automobiles were powered by hydrogen, since gasoline was considered too dangerous. During World War I and II, fuel shortages drove engineers to create hydrogen-powered vehicles. Today, major automobile manufacturers are building hydrogen fuel cell cars, which consume stored hydrogen gas (H2) in a fuel cell in order to produce electricity for an electric motor. But, there are a few challenges with using stored H2 as a fuel.
First of all, hydrogen gas is not energy-dense by volume, so a hydrogen fuel tank has to be huge, or it has to store hydrogen under extremely high pressure (70,000 psi) in order to be as energy-dense as diesel fuel. According to a Department of Transportation report titled, "Guidelines For Use of Hydrogen Fuel in Commercial Vehicles: Final Report," "Gaseous hydrogen fuel with the same amount of energy as one gallon of diesel fuel would only weigh about one third as much, but would occupy almost seven times the volume if stored at 10,000 psi." Second, there is no hydrogen infrastructure to fuel hydrogen-powered vehicles. While there are more than 70,000 diesel fuel stations across the country, there are less than 70 places in North America to buy hydrogen for automobiles. Commercially made hydrogen is produced by stripping the carbon atoms out of compressed natural gas (CNG) using steam. This process, known as cracking, converts CNG, which has a chemical formula of CH4, into hydrogen gas. So, unless one gets their energy to make hydrogen gas from wind, solar, or geothermal sources, they might as well just skip a step and burn the CNG.
Making Hydrogen From WaterElectrolysis is the scientific word describing what happens when electricity breaks the molecular bonds that hold water together. At the atomic level, water contains two atoms of hydrogen and one atom of oxygen. When two volts of electricity are passed through water, hydrogen bubbles form on the negative side of the circuit (cathode) and oxygen bubbles appear at the positive side of the circuit (anode). Hydrogen does not like to be alone, so it joins with oxygen right as it leaves the cell. This new gas is called oxyhydrogen (HHO). In the automotive world, the terms oxyhydrogen (HHO) and hydrogen are often used interchangeably, even though they are not technically the same thing.
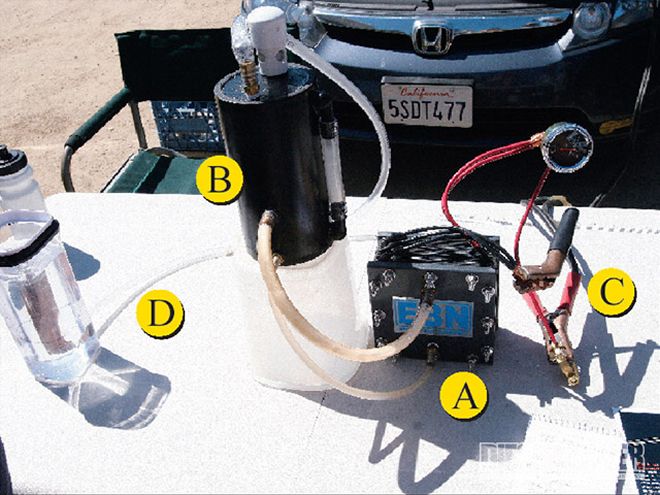
Hydrogen-On-DemandThe systems we are talking about in this article use the hydrogen-on-demand approach versus a high-pressure hydrogen-storage scheme. A hydrogen-on-demand system stores hydrogen gas compactly and safely as water until it is needed. One gallon of water equals about 1,800 gallons of HHO gas-this means you don't need a lot of water to make a lot of hydrogen. In most kits, the electricity used to power the cell that converts water into hydrogen comes from the vehicle's alternator and batteries.
Does it Really Work?Skeptics argue that the energy needed to separate water into hydrogen equals (or exceeds) the energy given off by burning HHO in the engine. There is also concern that enthusiasts and companies looking to sell hydrogen conversion kits oversimplify the process and do not take into account the advanced tuning needed to see results (especially with computer-controlled diesel vehicles). A quick search on Wikipedia nets different results depending on how you word it. For example, type in "water-fueled car" and it will say it doesn't work. Type in "hydrogen fuel enhancement" and the prospects brighten. The truth is, your results will depend on the efficiency of the HHO cell generator you use, and the efficiency of your engine. If our engines were more efficient, HHO wouldn't be needed in order to burn the primary fuel more completely. Remember, only about one third of the fuel you burn and convert into heat actually goes to powering the wheels, the rest is dissipated by the cooling system and exhaust. A good cell produces 3 liters of HHO gas a minute, which is still only enough hydrogen to run a lawn mower. This being the case, hydrogen-on-demand systems do not fuel the vehicle, instead the hydrogen is thought to speed up the combustion process. According to the guys at Hydrogen Hybrid Fuel Cell Corporation, as a rule of thumb, a hydrogen producing cell needs to produce half a liter per minute of H2 gas for each liter of displacement your diesel engine has.
Increasing EfficiencyAn acid, base, or salt can be added to the cell's water supply in order to drop the electrical resistance of the water. With a catalyst in the water, it takes fewer amps of electricity to produce the desired amount of HHO gas, since the ions in the water are able to move more freely. Potassium hydroxide (KOH) is a base and a popular catalyst for HHO-producing cells. It is important to make sure the catalyst you're using isn't highly concentrated, because it could damage your intercooler. This is an important aspect where more research is needed. Keep in mind, a properly designed cell will not need large doses of electrolyte. Varying amounts are added to the mixture in order to tune the onboard-hydrogen system. Heat, pressure, and electromagnetic fields are also employed to help break down the water, although this technology is not very mainstream.
HYDROGEN-ON-DEMAND DIRECTORYCanadian Hydrogen Energy Company LTD www.chechfi.com
Diamond Eye Performance www.diamondeyeperformance.com
EnergyBuilders.net www.energybuilders.net
Evergreen Hydrogen www.evergreenhydrogen.com
HHO Kit Store www.hhokitstore.com
GLOSSARYHydrogen - This is the first element in the periodic table. Two hydrogen atoms and one oxygen atom form water. If these same water molecules are rearranged, they can form a combustible gas.
Electrolysis - This is a process that uses electricity to rearrange water's molecular structure. Another name for this device is a hydrogen cell. Basically, water and electricity goes in, and flammable gas comes out.
HHO/oxyhydrogen - This is the result of electrolysis. Since hydrogen is not comfortable being by itself, it attaches to oxygen as soon as it leaves the cell. In the automotive world, when people say they are burning hydrogen in their car, they are really consuming HHO, or oxyhydrogen, another name for HHO.
Catalyst/electrolyte - In order to lower water's electrical resistance, a catalyst or electrolyte is used. They come in the form of an acid, base, or salt. Potassium hydroxide (KOH), a base, is a common catalyst used by enthusiasts.
SAFETY RULES1. Everything in your hydrogen injection system should be made from stainless steel, plastics, or alloys resistant to chemical erosion. It is important not to run copper wires because they will short out. Glass should absolutely be avoided.
2. A water reservoir, known as a bubbler, should be installed between the hydrogen-producing cell and engine along with a one-way valve in order to eliminate the possibility of an explosion going all the way to the cell.
3. Your cell should have a pressure-release valve built in.
4. Your electrolysis system needs a fuse, relay, power switch, and 12-volt ignition source (so the cell is deactivated once the engine is shut off).
5. The cell has the potential to produce lethal gases depending on the electrolyte, so beware.
6. Never, under any circumstances, store HHO or oxyhydrogen gas, since it is much less stable than pure hydrogen and can explode.
 | hydrogen Fuel hydrogen
"Run your car on water". "Clean the air as you drive". "Do it yourself, it's cheap and easy". More than likely, you've heard these claims discussed in conversations, posted on the web, or seen them in magazine ads. If not, let Diesel Power be the first to tell you that yes, water has the potential to help power your diesel vehicle. The question becomes: is hydrogen for you, and at what cost?
Hydrogen Basics
| hydrogen Fuel hydrogen
"Run your car on water". "Clean the air as you drive". "Do it yourself, it's cheap and easy". More than likely, you've heard these claims discussed in conversations, posted on the web, or seen them in magazine ads. If not, let Diesel Power be the first to tell you that yes, water has the potential to help power your diesel vehicle. The question becomes: is hydrogen for you, and at what cost?
Hydrogen Basics