

Many Pontiac owners just accept the fact that a high-horsepower engine is going to run hot on the street. Doug Tornello was one of them. His beautiful '67 GTO certainly fits the assumption. Sporting a 560hp, 510ci engine with an IA cast-iron block, aluminum cylinder heads, and 10.75:1 compression, all on 93-octane pump gas, this Poncho had the right to create an elevated coolant temperature if anything did.
The car had all the proper parts: a large Be Cool aluminum radiator and high-speed electric fan. However, around town and in traffic, the coolant would quickly go up to 240-plus degrees. Then the engine would run poorly, not only from the excessive heat but also the boiling of the fuel in the Demon carburetor. At a constant highway speed, the engine stayed cooler (200-210 degrees), but as soon as the GTO stopped moving, the gauge would spike up and stay there. This condition wasn't acceptable; Doug lived in worry of getting caught in a bad traffic jam, overheating and damaging the engine.
Diagnosing The Problem
The first step in reducing the coolant temperature of Doug's Pontiac is to diagnose the problem. The key element here is this Pontiac ran cooler at high speeds than around town and below 40 mph. A good rule in this scenario (assuming there isn't airflow restriction to the radiator) is to examine the water-pump flow rate.
Our subject Pontiac was equipped with an underdrive pulley system that featured a 6-inch-diameter water-pump pulley, which is larger than the stock Pontiac design. The larger pulley slowed the stock replacement water pump to the extent that at lower speeds there was insufficient coolant flow through the system, resulting in a higher coolant temperature. The first step was to locate a smaller (5-inch or less) water pump pulley.
When a search of the available parts was exhausted, Melvin Benzaquen of Classic Restoration Enterprises contacted Fred Bellscheidt of B&R Industries. This custom machine shop was able to create the desired smaller water-pump pulley to increase the coolant flow at low speeds. With the new pulley installed, the coolant temperature immediately dropped approximately 20 degrees around town and during slow driving, but the Pontiac still became ornery when it got too hot under the hood. There was a second component contributing to the problem.
Studying how an engine's cooling system works, you quickly see the traditional coolants, such as pure water or a mix of antifreeze and water, are much less than ideal. Though acceptable for a stock engine, one with elevated power needs more cooling efficiency than these liquids can handle. Evans Cooling Systems in Sharon, Connecticut, has developed a coolant called NPG+ (nonaqueaous propylene glycol) that doesn't use water, never freezes, and doesn't boil until 375 degrees. It's also a lifetime coolant, so maintenance is eliminated once it's installed. Before you can understand this completely new technology, a brief overview of coolant basics is in order.
(Editor's Note: The coolant basics portion of this article was excerpted by the author from his book, Engine Cooling Systems, from HP Books.)
The Attributes Of A Coolant
From the first use of water for cooling to today's modern engines, the basic elements that are essential for a liquid to function as an acceptable coolant have remained unchanged. In recent years, the list has grown even longer due to the different style of engines produced and the materials they use.
Let's first define the core characteristics of engine coolants and then expand into the requirements for a high-performance Pontiac engine.
As its name implies, the primary purpose of a liquid coolant is to remove heat from the cylinder head and engine block. Secondary concerns all apply to how the coolant transfers heat, its ability to be pumped, limit corrosion and deposits, offer lubricity and not freeze. As water freezes, it expands and would crack the castings of the engine if left unchecked.
Without getting mired too deep in chemistry, the basic terms for the qualities of a coolant are: molecular weight or solubility, hygroscopicity, vapor pressure and boiling point, viscosity, freeze point, specific heat, density, surface tension, air contact, flammability, refractive index and toxicity.
Traditional antifreeze is a 50/50 mix of ethylene glycol and water that is often abbreviated as EG.
Heat is transferred from the cylinder bore and cylinder-head walls to the liquid coolant via convection or semiconvection phases. These phases are dependent on the rate of heat flow through the metal-per-unit area along with the temperature difference between the metal surface and the liquid coolant. As the coolant reaches the hottest part of the cylinder head, which is usually around the combustion chamber and exhaust valve, it will actually start to boil. This phase change is identified as the nucleate boiling point and allows efficient transfer of heat. The coolant's chemical and thermal reaction are responsible for how efficient this process becomes.
When the coolant first comes in contact with the hot metal, it will boil, changing phase, and then due to the pressure in the cooling system, the gas bubbles will be pushed from the localized boiling spot and carry with it the heat. It then recondenses into a liquid.
The coolant can experience four distinct phase changes:
• Convection Phase
At very low rates of heat flow into the coolant, no boiling occurs, and the movement of the liquid is by free convection or by forced convection, caused by the water pump pressure. In a cooling system, the majority of the heat transferred from the cylinder bores to the coolant occurs by natural and forced convection currents where the heat flow through the metal is relatively low.
• Nucleate Boiling Phase
At higher engine loads and speeds, the rate of heat flow through the cylinder head is increased until steam bubbles are formed in certain regions on the surface of the water jacket. These areas are the metal bridge between the exhaust valve seats, spark-plug boss, and so on. Nucleate boiling involves the nucleation and growth of vapor bubbles that originate at sites of high temperature. In this phase, large numbers of bubbles form on the hot surfaces and travel through the bulk of the coolant, later recondensing as they move to a lower temperature region.
• Unstable Film Boiling Phase
Under severe engine load and speed, the vapor bubbles become so large and numerous that the liquid has difficulty flowing back to the hot metal surface of the cylinder head. When this critical heat flux is reached, the hot surface of the water jacket suddenly becomes insulated by individual steam bubbles, which join together to form a film. Under these conditions, the film insulates the water jacket from the coolant and metal surface temperature of the cylinder head elevates dramatically and can cause the engine to ping even though the temperature gauge shows no sign of this condition. This phase is often called crisis boiling.
• Stable Film Boiling Phase
If the metal surfaces subjected to unstable film boiling can withstand even higher heat levels without melting, the temperature is raised further by increasing the difference between the metal surface and the liquid boiling temperature, until a stable film is formed. With stable film boiling, heat transfer is now mainly by conduction and radiation across the vapor film. At this time, engine failure is very possible.
In every Pontiac engine the first two phases of coolant boiling occurs (in the cylinder head), while the last two will be experienced when either the horsepower level is too high for the coolant or the system is not designed properly.
 The Evans NPG+ is a gold color when new but over time will become very dark. When this happens, there is nothing wrong. All of the dyes used for antifreeze are water-based ,and since the system is non-aqueous, the dye dissipates.
The Evans NPG+ is a gold color when new but over time will become very dark. When this happens, there is nothing wrong. All of the dyes used for antifreeze are water-based ,and since the system is non-aqueous, the dye dissipates.
Vapor Pressure And Boiling Point
All liquids create vapors. The amount of vapor produced is defined by the chemical characteristics of the liquid. Pressure exerted by these vapors in the presence of the liquid is defined as vapor pressure, which increases as the temperature is elevated.
The boiling point of a liquid is defined as the temperature at which the vapor pressure is equal to the external pressure on the surface of the liquid. When a liquid is heated in an open vessel, it will boil when its vapor pressure is equal to the atmospheric pressure.
Keeping this in mind, as elevation is increased the atmospheric pressure decreases, and thus, the boiling point of a liquid is lower. Back in the early days, it was common for the engines to "boil over" when carrying a heavy load in high elevations, like those found in the mountains of the western United States. These early cooling systems were not pressurized, so increases in altitude were very problematic.
Glycols have lower vapor pressures than water and their boiling points are higher. If external pressure is reduced, the boiling point of the glycols will also be reduced but will still be higher than water. Thus, glycols are considered high-boiling-point liquids because of their low vapor pressure compared to that of water at a given temperature. As an example, at 68 degrees F the vapor pressure of water is more than 100 times greater than that of some glycols. The low volatility of glycols lessens their tendency to evaporate and has led to their use as antifreeze for engines.
Mixes of glycol and water generally have physical properties between those of water and anhydrous glycols. Adding water lowers its boiling point over that of straight glycol. The smaller the concentrate, the lower the boiling point will be.
Glycols are usually more fluid than many of the other high-boiling-point chemicals. For this reason, they work well when mixed with water to create a viscosity that can be pumped easily when cold through an engine, radiator and heater core.
 We used a Lisle Radiator Fill Kit to help bleed the cooling system. This is a great tool that belongs in every hobbyist's garage. It allows the cooling system to be filled and bled without spitting any coolant on the ground.
We used a Lisle Radiator Fill Kit to help bleed the cooling system. This is a great tool that belongs in every hobbyist's garage. It allows the cooling system to be filled and bled without spitting any coolant on the ground.
Freeze Point
When liquids are cooled, they eventually either crystallize like ice or become increasingly viscous (thicker) until they fail to flow and set up like glass. Crystallization represents true freezing that is seen with water, while a change into a glass-like substance is called supercooling.
Glycols do not have sharp freezing points. Under normal conditions, straight glycol would supercool rather than freeze.
Specific Heat
Specific heat is defined in the McGraw-Hill Dictionary of Engineering as: "The ratio of the amount of heat required to raise a mass of material one degree in temperature to the amount of heat required to raise an equal mass of a reference substance, usually water, 1 degree in temperature. Both measurements are made at a reference temperature usually at a constant pressure or constant volume."
In simpler terms, specific heat can be considered the ability of the liquid to store heat. The specific heat of water is approximately 1 at ordinary temperatures. Specific heat varies with temperature, so a value for a reference needs to be assigned.
The specific heats of glycol mixes are higher than straight glycols. This is important when engine coolants are used as antifreeze. A liquid with a high specific heat will do more work per unit weight than one with a low specific heat, if all other factors are equal.
Surface Tension
Surface tension is the force on the surface of a liquid, which tends to diminish the surface area to a minimum. The surface tensions of glycols are less than that of water. Generally, when heat is applied to a liquid, the surface tension is reduced. In the case of glycols, heat does not affect the surface tension materially, except when the temperature is near the boiling point.
 Taking a sample reading using a refract-ometer came next. The coolant from the engine is placed on the glass and the moisture content is measured in a scale identified as Brix.
Taking a sample reading using a refract-ometer came next. The coolant from the engine is placed on the glass and the moisture content is measured in a scale identified as Brix.
The scale that surface tension is measured in is dynes/centimeter. Water has a very high surface tension (72 dyn/cm), which makes it a poor choice for an engine coolant. A mix of EG and water (50/50) has a reading of 56 dyn/cm, while the Evans NPG+ is much easier to release at 44 dyn/cm.
The Boiling Cycle
The coolant used in an engine undergoes a boiling phase that is required to remove heat from the cylinder head. Up until very recently, the premise of most cooling system designs was to eliminate or not allow boiling to occur. In simple terms, the idea of having boiling coolant in an engine was thought of as the result of either poor engineering or a component failure. Those readers who are older will remember the dreaded "boilover" that would occur and leave motorists stranded on the side of the road, waiting for the engine to cool and figuring out how they would transport water from a nearby stream or lake into their vehicle's radiator.
When it comes to boiling, it's true that it's not desirable to have this occur in the radiator. The radiator's job is to cool the liquid, while the liquid's task is to cool the engine. If the radiator of an engine is boiling over, it means the coolant is becoming superheated and turning to a vapor, and when it recondenses, it contains too much heat for the radiator to dissipate. So as you can see, we need to identify when it is good and sound engineering to have the coolant boil.
Before this topic can be explored, it needs to be recognized that the load on the cooling system and the coolant itself are not the same under all driving conditions. At idle and light load, such as cruising down an interstate highway, the engine is not required to produce much power. At 65 mph, the power required to move the vehicle is only a fraction of what the engine can produce at maximum output. On the average, an older Pontiac only requires 25-30 hp to cruise on level ground at 65 mph. Since an internal-combustion engine is nothing more than a heat pump, the thermal load the liquid coolant and the radiator, is exposed to is proportional to the heat of the power produced. If the same vehicle is asked to climb a long grade at a steep angle, pull a trailer, or produce maximum power for passing or racing, then the load on the coolant and radiator increases.
A coolant must work to meet the transient needs of the engine. Many falsely believe that water is the best coolant since it has a specific heat of 1. That would be true if the engine that it was used in was going to do nothing but idle or never produce anywhere near maximum power during its lifetime. With a specific heat of 1 and good thermal conductivity qualities, water does an excellent job of cooling an engine; that is, until the load is increased and the coolant starts to boil in the cylinder heads. Never mind the fact that water creates rust and corrosion that insulates the liquid from the water passages, diminishing thermal transfer in the engine and radiator. When this happens in the engine, the water would stay cooler since there is less heat rejected into it, but the metal surface temperature of the cylinder head would be much higher. In addition, water offers no lubrication properties for the pumping mechanism (water pump) and will freeze when cooled.
Traditional antifreeze that is a mix of EG and water solves some of the problems of pure water, but brings along its own set of issues. Water by itself has a very high surface tension, and when it does boil, it's harder for it to release and recondense back to a liquid. Thus it has limited ability to remove heat from the boiling point area and follow the laws of physics.
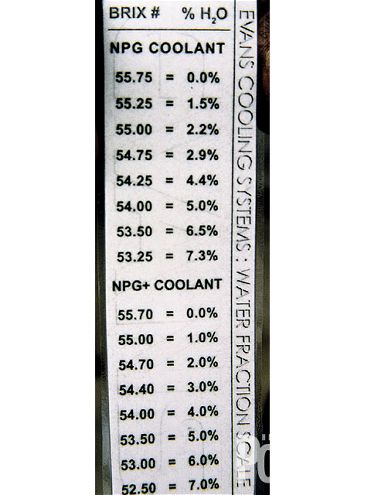 The ideal is to be less than 5 percent water on a refill such as ours. The GTO tested at 4 percent. At the time of publishing the prices were: Prep Fluid, $27.75/gallon; NPG+, $32.50/gallon; water pump, $189.95; thermostat, $9.95.
The ideal is to be less than 5 percent water on a refill such as ours. The GTO tested at 4 percent. At the time of publishing the prices were: Prep Fluid, $27.75/gallon; NPG+, $32.50/gallon; water pump, $189.95; thermostat, $9.95.
When water is employed as a coolant-a use that is common with competition and especially drag-race engines-the flow is usually restricted; the theory being, "slow the water down so that it has time to cool off in the radiator." This is an inaccurate response to the use of a poor coolant. To limit boilover, the higher pressure is required to raise the vapor point of the water to try to keep the cylinder head cool. When a restrictor is placed in the flow path, the system pressure increases and thus, the boiling point. In tests conducted by independent sources, some race engines had pressures as high as 60 psi or greater in the rear of the cylinder head because of the use of a flow restrictor. The engine builders thought they were controlling heat by reducing flow, but in actuality were creating such a high pressure to make up for the deficiencies of water as a coolant, even when treated with chemicals that are supposed to enhance its ability.
What is usually not taken into consideration is the added horsepower required to drive the water against 60 psi of pressure that the restrictor creates. These are all Band-Aid efforts to make up for the poor qualities of water as a coolant in anything other than an engine that is going to idle and produce only a minimum amount of its rated maximum power.
It is advantageous for the coolant to boil in the cylinder head, but it needs to refrain from undergoing a phase change for as long as possible for it to be the most effective. Then, it must release easily so it can move from that spot and take heat with it. Next, it's required to recondense quickly and be pumped with ease. Once it enters the radiator, it must possess enough thermal dissipation to drop in temperature and be ready for the entire event again.
As the coolant is asked to work harder, boiling occurs in the cylinder-head water jacket. Thus, the antifreeze can actually wear out. This is a result of the additive package becoming neutralized and consumed from the high temperatures and constant phase change that is occurring. An engine that is loafing for most of its life and hardly ever has the coolant work hard will have the additives stay active longer. For this reason, if traditional EG-based coolants are used, they need to be changed and renewed on a regular basis. The recommendation of a maximum of three years is only for an engine that sees normal duty. In severe usage, such as racing or towing, the coolant would need to be changed much more frequently.
Evans NPG+ has a lower surface tension than EG/water, boils at 375 degrees F at atmospheric pressure allowing the coolant to remove more heat from the cylinder head, and eliminates all water and the possibility of corrosion, along with cylinder liner cavitation in wet-sleeved race engines such as the big bore LS series.
Even with a 15 psi pressure cap, water boils at 257 degrees F(water/antifreeze boils at 264 degrees F at 15 psi) giving the Evans product an advantage of 105 degrees. When choosing a coolant for a high-performance Pontiac engine, you need to consider the worst-case conditions, not idle or light load. Traditional coolants are inferior when the load/horsepower increases.
System Approach
Though the Evans NPG+ can be installed in any Pontiac engine (stock or modified) with excellent results, our subject vehicle with 560 hp was an extreme example of a street car.
Evans Cooling Systems offers a complete cooling system package for Pontiac engines. It features a high-efficiency water pump with a scroll-style impeller and bleed orifice along with a high flow Robertshaw thermostat.
Conclusion
With the complete Evans system installed, the subject GTO now runs 192 degrees F on the highway during summer weather. While sitting in traffic, the temperature never goes above 210 degrees F and, more important, since the coolant is removing the heat from the engine, it runs great at any temperature. The loading up and poor drivability after elevated coolant temperature is now gone. Doug said that previously he would open the hood after a 25-mile run and it would feel like a blast furnace. With the Evans system, he told HPP that it stays much cooler under the hood.
The important thing to note is that though the liquid temperature on the gauge hasn't changed much aside from when the pulley was swapped for a smaller one, the cylinder-head metal surface temperature has dropped dramatically with the Evans NPG+. This proves the temperature-gauge reading is only part of the story when it comes to engine heat.
Now that the heat is under control, the next step is to put the Pontiac on the shop's chassis dyno, and recurve the distributor and lean the carburetor to take advantage of the cooler combustion chambers.
If you would never believe that a simple coolant swap would completely change how an engine runs, think again. With the Evans NPG+ this 560hp GTO runs as well on a 90-degree day as it did before in 60-degree weather. The key is keeping a cool head!