One of the hardest truths to swallow as a car owner is realizing that you’re going to spend time and money simply maintaining the damned thing. This is doubly painful for enthusiasts whose performance budgets are really just maintenance budget surpluses. While this month’s installment of Fact or Fiction doesn’t evaluatve products claiming to show you how best to (or not to) modify for performance, it does examine whether a questionable maintenance measure can save your bottom line, to help get you going faster sooner.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
The Claim: Battery Desulfators Can Bring Dead Batteries To Life.
When it comes to our cars’ electrical systems, most of us know just what we need to get by. This is especially true of battery maintenance: Keep your terminals clean, jumpstart it if you park with your lights on, let the alternator take care of the rest, and you’ll be good to go for a long while. Until the battery eventually refuses to hold a charge and you have to fork over hard-earned loot for a replacement, right? Not necessarily, say the makers of battery desulfators.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
Batteries come in many different shapes and sizes, are made of many different components, yet all serve the same fundamental purpose: storing and discharging electricity by converting chemical potential energy into electrical kinetic energy. The ones we’ll be concerning ourselves with this month are standard 12V lead/acid batteries like the one in your car. Essentially the world’s oldest rechargeable batteries, their construction and functionality are very simple: a positive terminal composed of lead dioxide and a negative terminal composed of porous lead are each immersed into electrolyte solutions consisting of water and sulfuric acid.
Chemical reactions between each terminal and its surrounding electrolyte solution produce a surplus of electrons in the negative side and a deficit of electrons in the positive side that prompts a flow of electrons from negative to positive once each side is connected through a circuit, when electricity is needed from the battery. This electro-chemical reaction produces a crystalline byproduct called lead sulfate, which collects on terminals in a process called sulfation. When your car’s alternator recharges the battery the process works in reverse, and the lead sulfate is reconverted into lead and sulfuric acid through a process called gassing (in which water is also converted into its essential elements of hydrogen and oxygen) before all components ideally return to their natural state.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
The problem is that we don’t live in an ideal world, and when a lead/acid battery is repeatedly overused or run dead, especially if it’s really old, lead sulfate ions can grow increasingly stronger bonds with the lead of the battery terminals, severely limiting the amount of voltage a battery can store and discharge. Manufacturers of battery desulfators claim that through controlled bursts of high voltage, their products can reverse even the most severe cases of sulfating, restoring battery performance to like-new condition.
To test their claims, we rounded up two popular battery desulfatorsVDC Electronics’ Battery Minder and the Xtreme Charge by Pulsetechand two seemingly dead test subject batteries: A run-of-the-mill Autolite 84, and a lightweight dry-cell Buddy Club Racing Spec Battery.
The first order of business is physical battery maintenance. Lead/acid batteries aren’t usually perfectly sealed, allowing the water in their electrolyte solution to evaporate over time. Make sure any battery you’re about to charge/restore is filled with the proper amount of clean, distilled water. Sulfation can spread to terminal posts; clean these off, too.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
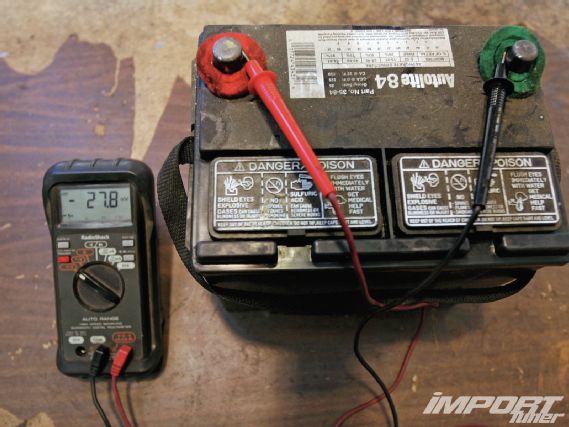
With our Autolite battery cleaned and filled, it still registered barely any charge—we had to switch our multimeter to millivolts for anything to register. Oh, and this was after it sat on a traditional charger for the better part of a day. Pretty much dead.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
Its voltage was so low that we had to actually jump it from another battery just to start the desulfation/restoration process with the Xtreme Charge.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
Average voltage while hooked up to the Xtreme Charge held at around 14 volts, though occasionally jumped much higher to facilitate the desulfation process. During three weeks of restoration, our Autolite was monitored using a voltmeter as the restoration process improved voltage to 11.38 volts, eventually finishing at 13.85, which it held for the following four months until we wrote this. We’ve driven it a lot during that time and also let it sit in our garage. For all intents and purposes, it’s been near-perfectly reclaimed.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
Next up was Buddy Club’s Racing Spec Battery, a sealed, Absorbed Glass Matt (AGM) battery commonly referred to as a “dry-cell” battery. It’s still a lead/acid type, but its semi-solid electrolyte replaces the water/sulfuric acid solution of traditional batteries like our Autolite. It’s still susceptible to sulfation; this particular one was drained and overcharged for years in competition before spending the subsequent four years abandoned on a garage floor. After spending an entire week on a traditional charger, it registered only 6.35 volts at rest—not nearly enough to crank over an engine.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
We hooked this one up to the Battery Minder. During its two-week test cycle, voltage rapidly fluctuates between 13.2 and 14.8 volts as the unit worked its magic to break down those sulfate bonds.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
After testing was complete, it held a consistent charge of 13.1 volts at rest and 14.1 running in our test Subaru STI. It, too, has upheld this performance for four months.
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
The Verdict: FACT
 |
Battery Desulfators - Fact or Fiction
|
Battery Desulfators - Fact or Fiction
The say the proof is in the pudding, but for us, it’s in the driving. With brand-new spares in tow, we drove with one of each reclaimed batteries under our hoods for months after our testing with no fault. Sure, their voltages aren’t exactly what they were sitting on the shelf at the speed shop all nice and new, but they’re a far cry from how we left them for dead. Battery desulfators win. We have no complaints. Other than not being able to find the receipts for the back-up batteries we bought . .

